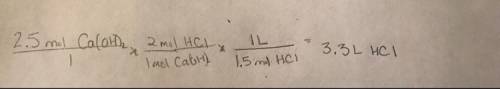Chemistry, 16.07.2019 08:30 weberalycia
How many liters of 1.5 m hcl solution would react completely with 2.5 moles ca(oh)2? (2 points) ca(oh)2(s) + 2 hcl(aq) > cacl2(aq) + 2 h2o(l) 0.83 l hcl(aq) 3.3 l hcl(aq) 1.9 l hcl(aq) 7.5 l hcl(aq)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:10
How many grams of naoh are needed to make 0.250 liter of a 0.500 m solution of naoh? 0.125 g 5.00 g 2.00 g
Answers: 1

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

You know the right answer?
How many liters of 1.5 m hcl solution would react completely with 2.5 moles ca(oh)2? (2 points) ca(...
Questions

Physics, 07.07.2019 18:00

English, 07.07.2019 18:00



History, 07.07.2019 18:00


French, 07.07.2019 18:00

Mathematics, 07.07.2019 18:00

English, 07.07.2019 18:00



Mathematics, 07.07.2019 18:00

English, 07.07.2019 18:00


History, 07.07.2019 18:00

Business, 07.07.2019 18:00

History, 07.07.2019 18:00

Mathematics, 07.07.2019 18:00