
Chemistry, 16.07.2019 04:00 cmfield8810
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain why the shift occurs. b. what would have been observed if hcl (also a strong acid) had been added instead of the hno3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
Hno3, a strong acid, is added to shift the ag2co3 equilibrium (equation 7.6) to the right. explain w...
Questions



Mathematics, 04.10.2019 21:40



Mathematics, 04.10.2019 21:40



Biology, 04.10.2019 21:40


Biology, 04.10.2019 21:40

Mathematics, 04.10.2019 21:40



Mathematics, 04.10.2019 21:40




Physics, 04.10.2019 21:40

Mathematics, 04.10.2019 21:40

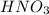 reacts with
reacts with 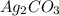 usually the equilibrium shifts to the right because
usually the equilibrium shifts to the right because 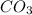 molecule from the reaction mixture, which then tends to shift the equilibrium towards right.
molecule from the reaction mixture, which then tends to shift the equilibrium towards right.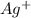 and
and 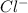 ions from the solution.
ions from the solution.

