Chemistry, 16.07.2019 02:00 madisonruh
Calculate the fraction of lattice sites that are schottky defects for cesium chloride at its melting temperature (645oc). assume an energy for defect formation of 1.86 ev.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
Calculate the fraction of lattice sites that are schottky defects for cesium chloride at its melting...
Questions











Advanced Placement (AP), 09.11.2019 03:31



History, 09.11.2019 03:31


Mathematics, 09.11.2019 03:31


Mathematics, 09.11.2019 03:31

Mathematics, 09.11.2019 03:31

Mathematics, 09.11.2019 03:31

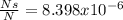
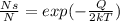
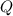 is the given energy in ev,
is the given energy in ev, 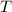 the temperature in Kelvins and
the temperature in Kelvins and 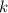 the Boltzmann's constant in ev/K, in this manner, the resulting fraction is shown below:
the Boltzmann's constant in ev/K, in this manner, the resulting fraction is shown below: