
Chemistry, 15.07.2019 22:00 eaglesjohnson414
Calculate the average reaction rate of cl2 consumed using the following information. h2 + cl2 yields 2 hcl a table with three columns and 3 rows. the first column is titled time. the second is titled the concentration of h subscript two (molar). the third column is titled the concentration of cl subscript two (molar). the first row shows 0.00 under time, 0.0505 under h subscript two and 0.0875 under cl subscript two. the second row shows 10.0 under time, 0.0255 under h subscript two and 0.0625 under cl subscript two. 6.81 × 10-1 mol/(l × s) 5.82 × 10-2 mol/(l × s) 7.50 × 10-2 mol/(l × s) 2.50 × 10-3 mol/(l × s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Calculate the average reaction rate of cl2 consumed using the following information. h2 + cl2 yields...
Questions



Computers and Technology, 29.07.2019 07:00

Mathematics, 29.07.2019 07:00

English, 29.07.2019 07:00


Mathematics, 29.07.2019 07:00

Social Studies, 29.07.2019 07:00




Spanish, 29.07.2019 07:00

History, 29.07.2019 07:00


Mathematics, 29.07.2019 07:00


Chemistry, 29.07.2019 07:00



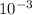 Mol/(L s)
Mol/(L s)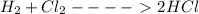 .
.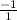
![\frac{delta [Cl]}{delta t}](/tpl/images/0093/9938/4c702.png) =
= 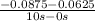 = 2.50 X
= 2.50 X 

