
Chemistry, 15.07.2019 21:30 paralaw61772
Calculate the mass of hcl required to prepare 2.5 liters of a 0.08 molar solution of hcl. 1 h 1.01 hydrogen 17 cl 35.45 chlorine a. 3.2 grams b. 4.5 grams c. 7.3 grams d. 11.4 grams

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Calculate the mass of hcl required to prepare 2.5 liters of a 0.08 molar solution of hcl. 1 h 1.01 h...
Questions

Mathematics, 15.01.2020 03:31

Mathematics, 15.01.2020 03:31


History, 15.01.2020 03:31





Physics, 15.01.2020 03:31

Mathematics, 15.01.2020 03:31





English, 15.01.2020 03:31

Biology, 15.01.2020 03:31

Computers and Technology, 15.01.2020 03:31

Mathematics, 15.01.2020 03:31


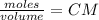
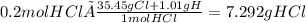 it's the choice c
it's the choice c

