
Chemistry, 15.07.2019 20:30 Badbpyz7550
Calculate the heat released when 5.00 l of cl2(g) with a density of 1.88 g/l reacts with an excess of sodium metal at 25°c and 1 atm to form sodium chloride.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
Calculate the heat released when 5.00 l of cl2(g) with a density of 1.88 g/l reacts with an excess o...
Questions



English, 19.05.2020 02:19


Mathematics, 19.05.2020 02:19

Biology, 19.05.2020 02:19

Mathematics, 19.05.2020 02:19


Mathematics, 19.05.2020 02:19

History, 19.05.2020 02:20


Mathematics, 19.05.2020 02:20


Mathematics, 19.05.2020 02:20





Social Studies, 19.05.2020 02:20

Biology, 19.05.2020 02:20

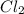 which has density of 1.88 g
which has density of 1.88 g

