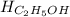Chemistry, 15.07.2019 19:30 CoolRahim9090
Calculate the standard molar enthalpy for the complete combustion of liquid ethanol (c2h5oh) using the standard enthalpies of formation of the reactants and products.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Calculate the standard molar enthalpy for the complete combustion of liquid ethanol (c2h5oh) using t...
Questions

Mathematics, 08.02.2021 17:40

Social Studies, 08.02.2021 17:40

History, 08.02.2021 17:40

History, 08.02.2021 17:40

Social Studies, 08.02.2021 17:40

History, 08.02.2021 17:40




Mathematics, 08.02.2021 17:40

Mathematics, 08.02.2021 17:40

World Languages, 08.02.2021 17:40



Physics, 08.02.2021 17:40

Social Studies, 08.02.2021 17:40



Computers and Technology, 08.02.2021 17:40

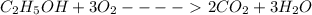
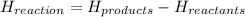
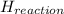 =
=