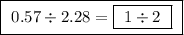Chemistry, 15.07.2019 17:30 Nanamoney5385
Achemist finds that 30.82 g of nitrogen will react with 17.60 g, 35.20 g, 70.40 g, or 88.00 g of oxygen to form four different compounds. calculate the mass of oxygen per gram of nitrogen in each compound.


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
Achemist finds that 30.82 g of nitrogen will react with 17.60 g, 35.20 g, 70.40 g, or 88.00 g of oxy...
Questions




English, 07.07.2019 00:30

Spanish, 07.07.2019 00:30

Mathematics, 07.07.2019 00:30


History, 07.07.2019 00:30



Geography, 07.07.2019 00:30

English, 07.07.2019 00:30

Mathematics, 07.07.2019 00:30

Mathematics, 07.07.2019 00:30

History, 07.07.2019 00:30

Mathematics, 07.07.2019 00:30

History, 07.07.2019 00:30

Biology, 07.07.2019 00:30

History, 07.07.2019 00:30

Mathematics, 07.07.2019 00:30

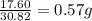 of oxygen
of oxygen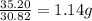 of oxygen
of oxygen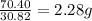 of oxygen
of oxygen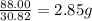 of oxygen
of oxygen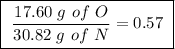
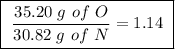
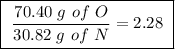
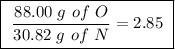
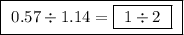 Between the second and third compounds:
Between the second and third compounds: 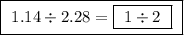 Between the first and third compounds:
Between the first and third compounds: