
Chemistry, 15.07.2019 17:00 ineedhelp2285
Calculate the pressure of o2 (in atm) over a sample of nio at 25.00°c if δg o = 212 kj/mol for the reaction. for this calculation, use the value r = 8.3144 j/k·mol. nio(s) ⇌ ni(s) + 1 2 o2(g)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
Calculate the pressure of o2 (in atm) over a sample of nio at 25.00°c if δg o = 212 kj/mol for the r...
Questions

Mathematics, 28.10.2020 05:30


English, 28.10.2020 05:30

Chemistry, 28.10.2020 05:30


Mathematics, 28.10.2020 05:30

Business, 28.10.2020 05:30

History, 28.10.2020 05:30


English, 28.10.2020 05:30

Computers and Technology, 28.10.2020 05:30



Law, 28.10.2020 05:30

Business, 28.10.2020 05:30


Physics, 28.10.2020 05:30

Mathematics, 28.10.2020 05:30

English, 28.10.2020 05:30

Mathematics, 28.10.2020 05:30

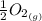 We can find out the pressure on oxygen by using the equation of gibb's free energy with remainder quotient which is, ΔG = ΔG° +RT lnQ
We can find out the pressure on oxygen by using the equation of gibb's free energy with remainder quotient which is, ΔG = ΔG° +RT lnQ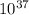
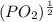 So, (1.51 X
So, (1.51 X  = 3.87 X
= 3.87 X 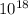
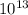 atm
atm

