

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 09:30
How many significant figures are in the following numbers ? a. 0.0002030 b. 2.000 c. 2.008900 d. 145.00
Answers: 2
You know the right answer?
Calculate the pressure of o2 (in atm) over a sample of nio at 25.00°c if δg o= 212 kj/mol for the re...
Questions


Mathematics, 06.05.2021 16:50

Biology, 06.05.2021 16:50





Computers and Technology, 06.05.2021 16:50

English, 06.05.2021 16:50

Mathematics, 06.05.2021 16:50

Chemistry, 06.05.2021 16:50

Computers and Technology, 06.05.2021 16:50


Mathematics, 06.05.2021 16:50

English, 06.05.2021 16:50

Mathematics, 06.05.2021 16:50

Chemistry, 06.05.2021 16:50


Mathematics, 06.05.2021 16:50

Mathematics, 06.05.2021 16:50

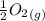
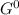 = 212 KJ/mol
= 212 KJ/mol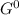 +RTlnQ
+RTlnQ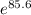 = 1.51 X
= 1.51 X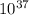
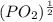
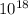 Pa
Pa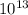 atm
atm

