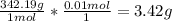Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
First: calculate the number of moles of kool-aid powder needed to make 100ml of a 0.1m solution. 0....
Questions


History, 01.11.2020 03:50


Chemistry, 01.11.2020 03:50

Chemistry, 01.11.2020 03:50

Mathematics, 01.11.2020 04:00

Mathematics, 01.11.2020 04:00





Mathematics, 01.11.2020 04:00

Social Studies, 01.11.2020 04:00





Biology, 01.11.2020 04:00

Mathematics, 01.11.2020 04:00

English, 01.11.2020 04:00

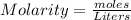
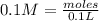
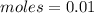
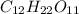 .
.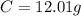
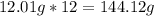
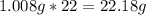
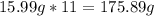
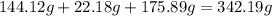 -> So we know that the molar weight of the Kool-Aid powder is 342.19g.
-> So we know that the molar weight of the Kool-Aid powder is 342.19g.