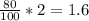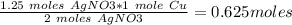Chemistry, 15.07.2019 10:30 Andrebutrus
#3.) hydrogen and oxygen react to produce water, according to the reaction 2h2 (g) + o2 (g) → 2h2o (l). if 3 moles of hydrogen react with 2 moles of oxygen and the yield in the reaction is 80%, how many moles of water are obtained? select one: a. 2 moles b. 2.4 moles c. 2.7 moles d. 3 moles#4.) which of the following is true about the total number of reactants and the total number of products in the reaction shown below? c5h12(l) + 8o2(g) → 5co2(g) + 6h2o(g)select one: a. 9 moles of reactants chemically change into 11 moles of product. b. 9 grams of reactants chemically change into 11 grams of product. c. 9 liters of reactants chemically change into 11 liters of product. d. 9 atoms of reactants chemically change into 11 atoms of product.#9.) how many moles of cu are needed to react with 1.23 moles of agno3? cu + 2agno3 → cu(no3)2 + 2agselect one: a. 0.62b. 2.5c. 3.51d. 0.55

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2


Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
#3.) hydrogen and oxygen react to produce water, according to the reaction 2h2 (g) + o2 (g) → 2h2o (...
Questions

Chemistry, 17.07.2019 01:30


History, 17.07.2019 01:30


French, 17.07.2019 01:30

Mathematics, 17.07.2019 01:30

Physics, 17.07.2019 01:30

Chemistry, 17.07.2019 01:30

Mathematics, 17.07.2019 01:30

Mathematics, 17.07.2019 01:30

Biology, 17.07.2019 01:30

Mathematics, 17.07.2019 01:30



English, 17.07.2019 01:30

History, 17.07.2019 01:30

Biology, 17.07.2019 01:30