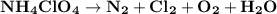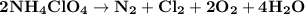Chemistry, 15.07.2019 10:00 angelaencinas90
Ammonium perchlorate nh4clo4 is a powerful solid rocket fuel, used in the space shuttle boosters. it decomposes into nitrogen n2 gas, chlorine cl2 gas, oxygen o2 gas and water vapor, releasing a great deal of energy. calculate the moles of ammonium perchlorate needed to produce 0.10mol of chlorine. be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1


Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1
You know the right answer?
Ammonium perchlorate nh4clo4 is a powerful solid rocket fuel, used in the space shuttle boosters. it...
Questions


Social Studies, 29.07.2019 06:00



Mathematics, 29.07.2019 06:00

Mathematics, 29.07.2019 06:00


Mathematics, 29.07.2019 06:00

Mathematics, 29.07.2019 06:00

Mathematics, 29.07.2019 06:00








English, 29.07.2019 06:00