

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2
You know the right answer?
How many moles of ag can be produced if 350. g of cu are reacted with excess agno3 according to the...
Questions



Mathematics, 10.09.2021 06:00

Chemistry, 10.09.2021 06:00

Mathematics, 10.09.2021 06:00


Mathematics, 10.09.2021 06:00

English, 10.09.2021 06:00

Physics, 10.09.2021 06:00

History, 10.09.2021 06:00

Mathematics, 10.09.2021 06:00


Mathematics, 10.09.2021 06:00



Mathematics, 10.09.2021 06:00

Mathematics, 10.09.2021 06:00


History, 10.09.2021 06:00

History, 10.09.2021 06:00

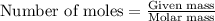
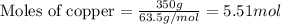
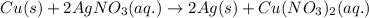
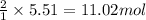 of silver.
of silver.

