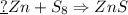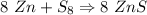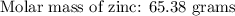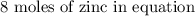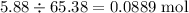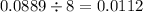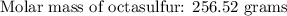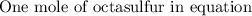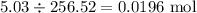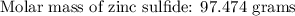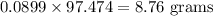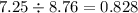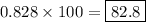Chemistry, 15.07.2019 05:00 xxaurorabluexx
If 5.88 grams of zn react with 5.03 grams of s8 to produce 7.25 grams of zns, what are the theoretical yield and percent yield of this reaction? be sure to show the work that you did to solve this problem. unbalanced equation: zn + s8 yields zns

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
If 5.88 grams of zn react with 5.03 grams of s8 to produce 7.25 grams of zns, what are the theoretic...
Questions



Mathematics, 03.01.2020 02:31

Geography, 03.01.2020 02:31

Mathematics, 03.01.2020 02:31

Spanish, 03.01.2020 02:31

Mathematics, 03.01.2020 02:31





English, 03.01.2020 02:31

English, 03.01.2020 02:31


History, 03.01.2020 02:31

Mathematics, 03.01.2020 02:31




Mathematics, 03.01.2020 02:31