Chemistry, 15.07.2019 03:00 hdhdenjdxk
What volume of a 2.75 m solution of naoh is required to make 500.0 ml of a 1.27 m solution of naoh?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
What volume of a 2.75 m solution of naoh is required to make 500.0 ml of a 1.27 m solution of naoh?...
Questions




Mathematics, 12.02.2021 14:00


Spanish, 12.02.2021 14:00



Mathematics, 12.02.2021 14:00

Advanced Placement (AP), 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

History, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00



Mathematics, 12.02.2021 14:00


Chemistry, 12.02.2021 14:00

Biology, 12.02.2021 14:00

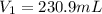
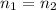
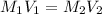
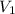 as it is the volume we need to take from the inital 2.75M solution of NaOH, thus:
as it is the volume we need to take from the inital 2.75M solution of NaOH, thus: