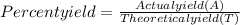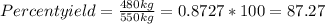Chemistry, 14.07.2019 23:30 breemills9953
The theoretical yield of ammonia in an industrial synthesis was 550 kg, but only 480 kg was obtained. what was the percentage yield of the reaction?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1


Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1
You know the right answer?
The theoretical yield of ammonia in an industrial synthesis was 550 kg, but only 480 kg was obtained...
Questions


Mathematics, 20.01.2021 22:50

Mathematics, 20.01.2021 22:50


English, 20.01.2021 22:50

Mathematics, 20.01.2021 22:50

Social Studies, 20.01.2021 22:50

Mathematics, 20.01.2021 22:50

Chemistry, 20.01.2021 22:50


English, 20.01.2021 22:50

Mathematics, 20.01.2021 22:50


Computers and Technology, 20.01.2021 22:50





Mathematics, 20.01.2021 22:50

Mathematics, 20.01.2021 22:50