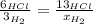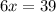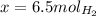Chemistry, 14.07.2019 22:30 maryalice2002
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can be formed? 2 al + 6hcl → 2 alcl3 + 3 h2 select one: a. al is the limiting reactant, 9.0 mol h2 can be formed b. hcl is the limiting reactant, 6.5 mol h2 can be formed c. al is the limiting reactant, 6.0 mol h2 can be formed d. hcl is the limiting reactant, 4.3 mol h2 can be formed

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
When 6.0 mol al react with 13 mol hcl, what is the limiting reactant, and how many moles of h2 can b...
Questions

Mathematics, 13.04.2021 05:40

Mathematics, 13.04.2021 05:40

Mathematics, 13.04.2021 05:40

Spanish, 13.04.2021 05:40


Mathematics, 13.04.2021 05:40

Mathematics, 13.04.2021 05:40

Mathematics, 13.04.2021 05:40


English, 13.04.2021 05:40


Mathematics, 13.04.2021 05:40

Mathematics, 13.04.2021 05:40

Business, 13.04.2021 05:40






Mathematics, 13.04.2021 05:40

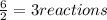
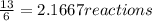
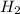 that are produced with 13 moles of HCl.
that are produced with 13 moles of HCl.