Chemistry, 14.07.2019 21:00 dbzrules02
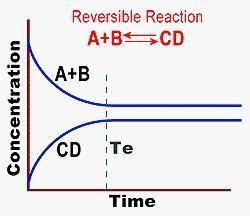
After reaching equilibrium, the rate of formation of products is less than the rate of formation of reactants. after reaching equilibrium, the rate of forming products and reactants is the same. equilibrium is obtained prior to te.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1
You know the right answer?
After reaching equilibrium, the rate of formation of products is less than the rate of formation of...
Questions


History, 08.04.2021 20:40




Mathematics, 08.04.2021 20:40

Mathematics, 08.04.2021 20:40




Mathematics, 08.04.2021 20:40

Mathematics, 08.04.2021 20:40


Mathematics, 08.04.2021 20:40



Mathematics, 08.04.2021 20:40

History, 08.04.2021 20:40