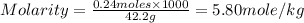Chemistry, 14.07.2019 19:30 MarishaTucker
Calculate the molality of a solution containing 14.3 g of nacl in 42.2 g of water. a. 2.45 ï´ 10â4 m b. 5.80 ï´ 10â4 m c. 2.45 ï´ 10â1 m d. 103 m e. 5.80 m

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
Calculate the molality of a solution containing 14.3 g of nacl in 42.2 g of water. a. 2.45 ï´ 10â4 m...
Questions


Mathematics, 19.11.2020 14:00


History, 19.11.2020 14:00



Mathematics, 19.11.2020 14:00



Mathematics, 19.11.2020 14:00



Mathematics, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00

Health, 19.11.2020 14:00



Advanced Placement (AP), 19.11.2020 14:00

Mathematics, 19.11.2020 14:00

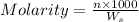
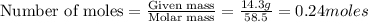
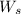 = weight of solvent in g= 42.2 g
= weight of solvent in g= 42.2 g