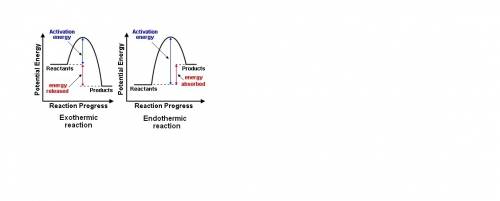
How does the potential-energy diagram for a reaction indicate whether the reaction is endothermic or exothermic? an endothermic reaction has reactants that are lower in energy than products because energy is absorbed to form the products. an endothermic reaction has reactants that are higher in energy than products because energy is released to form the products. an exothermic reaction has reactants that are lower in energy than products because energy is released to form the products. an exothermic reaction has reactants that are higher in energy than products because energy is absorbed to form the products.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
How does the potential-energy diagram for a reaction indicate whether the reaction is endothermic or...
Questions

Biology, 16.04.2021 22:00

History, 16.04.2021 22:00


Mathematics, 16.04.2021 22:00


History, 16.04.2021 22:00

Advanced Placement (AP), 16.04.2021 22:00

Mathematics, 16.04.2021 22:00

Mathematics, 16.04.2021 22:00


Mathematics, 16.04.2021 22:00




Mathematics, 16.04.2021 22:00

English, 16.04.2021 22:00

Spanish, 16.04.2021 22:00