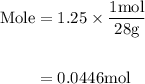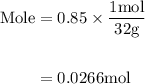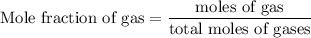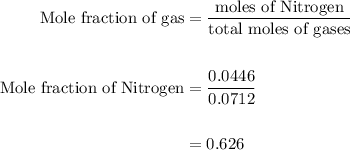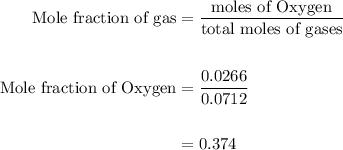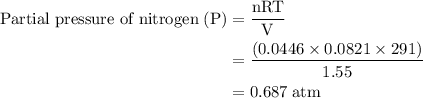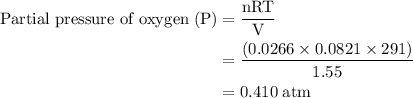Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
Agas mixture contains 1.25 g n2 and 0.85 g o2 in a 1.55 l c ontainer at 18 °c. calculate the mole fr...
Questions


Mathematics, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30


English, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30


Law, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30


Biology, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30

Social Studies, 10.11.2020 02:30


English, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30

English, 10.11.2020 02:30

History, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30