Chemistry, 14.07.2019 18:00 dootdootkazoot
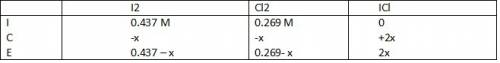
Consider the reaction between iodine gas and chlorine gas to form iodine monochloride. a reaction mixture at 298.15k initially contains i2 =0.437m and ci2=0.269m. what is concentration of ici when reaches equilibrium? keq= 81.9 @298.15k

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2


Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Consider the reaction between iodine gas and chlorine gas to form iodine monochloride. a reaction mi...
Questions

Mathematics, 02.04.2020 19:08

Mathematics, 02.04.2020 19:09

Biology, 02.04.2020 19:09

Mathematics, 02.04.2020 19:09

Mathematics, 02.04.2020 19:09



Mathematics, 02.04.2020 19:09


Mathematics, 02.04.2020 19:09

Mathematics, 02.04.2020 19:09


Mathematics, 02.04.2020 19:09


History, 02.04.2020 19:10






![K_{eq} = \frac{[ICl]^{2}}{[I_{2}][Cl_{2}]}](/tpl/images/0089/5177/ae97d.png)
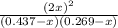
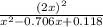
![(2x)^{2} = 81.9 [ x^{2} -0.706x + 0.118]](/tpl/images/0089/5177/63f20.png)
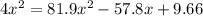
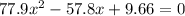
![[ICl]_{eq} = 2 ( 0.254) = 0.508 M](/tpl/images/0089/5177/bbf8d.png)