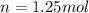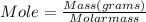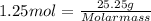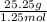Chemistry, 14.07.2019 17:30 EllaLovesAnime
Anoble gas is placed in a 10.0 liter container. the noble gas has a mass of 25.25 g, the pressure is 3.05 atm, and the temperature is 24°c. find the molar mass and symbol for the gas.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2


Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
Anoble gas is placed in a 10.0 liter container. the noble gas has a mass of 25.25 g, the pressure is...
Questions

English, 13.02.2020 23:49


Mathematics, 13.02.2020 23:49

Mathematics, 13.02.2020 23:49





Mathematics, 13.02.2020 23:49




English, 13.02.2020 23:49







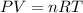
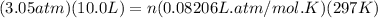
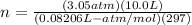 ... Unit K gets cancelled
... Unit K gets cancelled