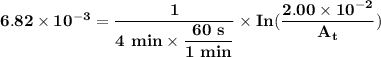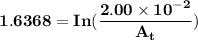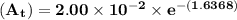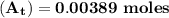Chemistry, 14.07.2019 17:30 guccikathyyy6195
The first-order rate constant for the decomposition of n2o5, 2n2o5(g)→4no2(g)+o2(g) at 70∘c is 6.82×10−3 s−1. suppose we start with 2.00×10−2 mol of n2o5(g) in a volume of 2.3 l . you may want to reference (page) section 14.4 while completing this problem. part a how many moles of n2o5 will remain after 4.0 min ? ,

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
The first-order rate constant for the decomposition of n2o5, 2n2o5(g)→4no2(g)+o2(g) at 70∘c is 6.82×...
Questions

Chemistry, 26.01.2020 02:31

Biology, 26.01.2020 02:31



Mathematics, 26.01.2020 02:31


Mathematics, 26.01.2020 02:31



Physics, 26.01.2020 02:31

Mathematics, 26.01.2020 02:31

History, 26.01.2020 02:31

Mathematics, 26.01.2020 02:31


Mathematics, 26.01.2020 02:31


Mathematics, 26.01.2020 02:31

Chemistry, 26.01.2020 02:31


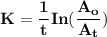
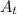 = ???
= ???