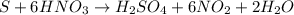Chemistry, 14.07.2019 16:30 AnaiyaKirksey8
How many grams of no2 are theoretically produced if we start with 1.20 moles of s and 9.90 moles of hno3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1
You know the right answer?
How many grams of no2 are theoretically produced if we start with 1.20 moles of s and 9.90 moles of...
Questions



Social Studies, 05.11.2021 20:30


History, 05.11.2021 20:30



Computers and Technology, 05.11.2021 20:30


Mathematics, 05.11.2021 20:30

Mathematics, 05.11.2021 20:30

Arts, 05.11.2021 20:30


Social Studies, 05.11.2021 20:40

Mathematics, 05.11.2021 20:40

Mathematics, 05.11.2021 20:40



Mathematics, 05.11.2021 20:40

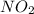 are formed.
are formed.