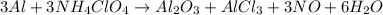Chemistry, 14.07.2019 15:00 franky2871
The reusable booster rockets of the u. s. space shuttle employ a mixture of aluminum and ammonium perchlorate for fuel. a possible equation for this reaction is3al1s213nh4clo41s2hal2o31s21alcl3 1s213no1g216h2o1g2what mass of nh4clo4 should be used in the fuel mixture for every kilogram of al?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
The reusable booster rockets of the u. s. space shuttle employ a mixture of aluminum and ammonium pe...
Questions


Mathematics, 27.01.2021 05:30

English, 27.01.2021 05:30


Arts, 27.01.2021 05:30


Mathematics, 27.01.2021 05:30

Social Studies, 27.01.2021 05:30

Mathematics, 27.01.2021 05:30





Biology, 27.01.2021 05:30






Mathematics, 27.01.2021 05:30