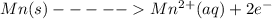Chemistry, 14.07.2019 14:30 sciencecreation87
An electrochemical cell has the following overall reaction: mn(s) + cu2+(aq) > cu(s) + mn2+(aq) the reduction half-reaction has a standard potential of 1.18 v. the oxidation half-reaction has a standard potential of 0.34 v. what is the overall cell potential? (a) -1.52 v (b) -1.18 v (c) +1.18 v (d) +1.52 v

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1

Chemistry, 23.06.2019 12:30
Growing crops in places where major pests don't live using beneficial insects to eat harmful insects using a rat trap instead of a rodenticide developing drought-resistant tomato plants using beneficial insects or natural oils to repel pests planting a different crop every year to fake out the pests keeping food covered to deter ants and rodents developing bean plants with a resistance to fungus picking caterpillars off tomato plants cultivation practice biological control cultural control genetic resistance natural chemicals
Answers: 3
You know the right answer?
An electrochemical cell has the following overall reaction: mn(s) + cu2+(aq) > cu(s) + mn2+(aq)...
Questions

English, 24.06.2019 21:10


Spanish, 24.06.2019 21:10


English, 24.06.2019 21:10

Mathematics, 24.06.2019 21:10





Biology, 24.06.2019 21:10

Mathematics, 24.06.2019 21:10

Mathematics, 24.06.2019 21:10

English, 24.06.2019 21:10

Mathematics, 24.06.2019 21:10





Social Studies, 24.06.2019 21:10