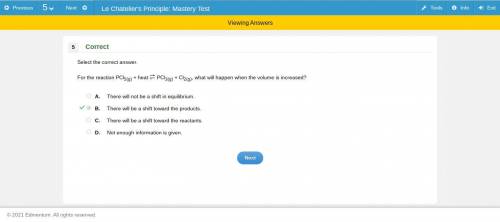
Chemistry, 14.07.2019 13:30 meganjackson155
For the reaction pcl5(g) + heat pcl3(g) + cl2(g), what will happen when the volume is increased? a. there will not be a shift in equilibrium. b. there will be a shift toward the products. c. there will be a shift toward the reactants. d. not enough information is given. if you can figure out the answer, you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
For the reaction pcl5(g) + heat pcl3(g) + cl2(g), what will happen when the volume is increased? a....
Questions


History, 16.11.2020 04:30

Chemistry, 16.11.2020 04:30






Social Studies, 16.11.2020 04:30











English, 16.11.2020 04:30