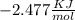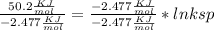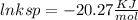Chemistry, 14.07.2019 13:30 Yorlin4441
Use the thermodynamic data at 298 k below to determine the ksp for barium carbonate, baco3 at this temperature. substance: ba2+(aq) co32–(aq) baco3(s) δh°f (kj/mol): –538.36 –676.26 –1219 δg°f (kj/mol): –560.7 –528.1 –1139 s°(j/k·mol): 13 –53.1 112

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
Use the thermodynamic data at 298 k below to determine the ksp for barium carbonate, baco3 at this t...
Questions



History, 05.03.2020 00:20

Mathematics, 05.03.2020 00:20

English, 05.03.2020 00:20

English, 05.03.2020 00:20

Biology, 05.03.2020 00:20











English, 05.03.2020 00:22

Computers and Technology, 05.03.2020 00:22

English, 05.03.2020 00:22

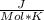
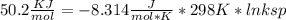
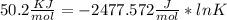
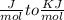
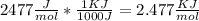 ))
))