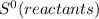Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Aluminum forms a layer of aluminum oxide when exposed to air which protects the bulk metal from furt...
Questions




Mathematics, 22.12.2020 21:40

Computers and Technology, 22.12.2020 21:40

Business, 22.12.2020 21:40

History, 22.12.2020 21:40


History, 22.12.2020 21:40

Mathematics, 22.12.2020 21:40

Health, 22.12.2020 21:40


Mathematics, 22.12.2020 21:40

English, 22.12.2020 21:40


Mathematics, 22.12.2020 21:40

Mathematics, 22.12.2020 21:50




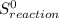 = ΣnΔ
= ΣnΔ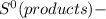 Δ∑n
Δ∑n