
Chemistry, 14.07.2019 13:00 devenybates
At which temperature would a reaction with h = -92 kj/mol, s = -0.199 kj/(molk) be spontaneous?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
At which temperature would a reaction with h = -92 kj/mol, s = -0.199 kj/(molk) be spontaneous?...
Questions

Mathematics, 26.08.2019 22:00


Mathematics, 26.08.2019 22:00

Mathematics, 26.08.2019 22:00

History, 26.08.2019 22:00




Physics, 26.08.2019 22:00

English, 26.08.2019 22:00

Health, 26.08.2019 22:00




Computers and Technology, 26.08.2019 22:10





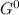 = Δ
= Δ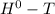 Δ
Δ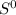
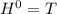 Δ
Δ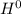 , giving a negative value for Δ
, giving a negative value for Δ 


