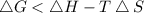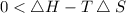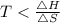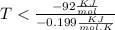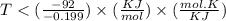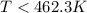Chemistry, 14.07.2019 12:30 natishtaylor1p8dirz
At which temperature would a reaction with h -92 kj/mol, s -0.199 kj/(mol-k) be spontaneous? a.600k b.500k c.400k d.700k

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1


Chemistry, 23.06.2019 18:10
Which is an aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas? particle spacing can allow a very high density. particle kinetic energy is independent of temperature. particles vibrate quickly in stationary positions. particles exchange energy through elastic collisions.
Answers: 2
You know the right answer?
At which temperature would a reaction with h -92 kj/mol, s -0.199 kj/(mol-k) be spontaneous? a.600k...
Questions

English, 24.06.2019 01:00

History, 24.06.2019 01:00

History, 24.06.2019 01:00

English, 24.06.2019 01:00

Mathematics, 24.06.2019 01:00

History, 24.06.2019 01:00

Social Studies, 24.06.2019 01:00


Mathematics, 24.06.2019 01:00

Mathematics, 24.06.2019 01:00

English, 24.06.2019 01:00



History, 24.06.2019 01:00

Physics, 24.06.2019 01:00





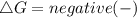
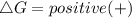
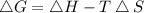
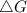 should be negative , so the above formula can be written as :
should be negative , so the above formula can be written as :