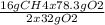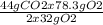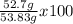Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 and 78.3g o2 to react. when the reaction is finished, the chemist collects 52.7g co2. determine the limiting reagent, theoretical yield, and percent yield for the reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Consider the following reaction. ch4 (g) + o2 (g) co2 (g) + h2o (l) a chemist allows 23.2g of ch4 an...
Questions

Chemistry, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40

History, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40


Mathematics, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40


Advanced Placement (AP), 12.03.2021 19:40


Mathematics, 12.03.2021 19:40




Biology, 12.03.2021 19:40