Chemistry, 14.07.2019 12:00 tybreyonnaHco7855
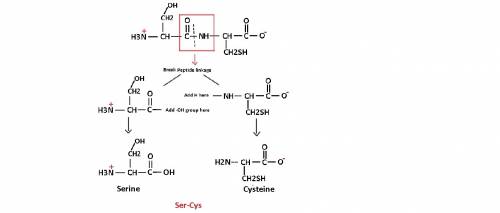
This dipeptide is designated as: there is a structure for h3nchcnhchco with ch2oh group attached to the first (from left to right) carbon, two oxygen atoms attached to the second and fourth carbons by double bonds, and ch2sh group attached to the third carbon. nitrogen has a charge of 1 plus. oxygen atom, which is attached to the fourth carbon by a single bond, has a charge of 1 minus.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


You know the right answer?
This dipeptide is designated as: there is a structure for h3nchcnhchco with ch2oh group attached to...
Questions

English, 19.11.2020 19:40

History, 19.11.2020 19:40

History, 19.11.2020 19:40

Mathematics, 19.11.2020 19:40

Mathematics, 19.11.2020 19:40



Chemistry, 19.11.2020 19:40





Spanish, 19.11.2020 19:40


English, 19.11.2020 19:50




Mathematics, 19.11.2020 19:50