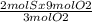Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
Consider the reaction between reactants s and o2: 2s(s)+3o2(g)→2so3(g)if a reaction vessel initiall...
Questions



Mathematics, 30.10.2020 03:30



Mathematics, 30.10.2020 03:30


Mathematics, 30.10.2020 03:30

Mathematics, 30.10.2020 03:30

Mathematics, 30.10.2020 03:30

Chemistry, 30.10.2020 03:30

Biology, 30.10.2020 03:30

Mathematics, 30.10.2020 03:30

History, 30.10.2020 03:30


Mathematics, 30.10.2020 03:30



Mathematics, 30.10.2020 03:30

Medicine, 30.10.2020 03:30