
Chemistry, 14.07.2019 10:30 kiannasmith46
Ammonia gas and oxygen gas react to form water vapor and nitrogen monoxide gas. what volume of nitrogen monoxide would be produced by this reaction if of oxygen were consumed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3


You know the right answer?
Ammonia gas and oxygen gas react to form water vapor and nitrogen monoxide gas. what volume of nitro...
Questions

Spanish, 03.03.2020 02:39





Computers and Technology, 03.03.2020 02:39








Computers and Technology, 03.03.2020 02:40




Computers and Technology, 03.03.2020 02:40


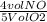 x 6.3 L
x 6.3 L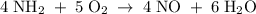
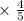 L
L

