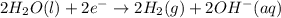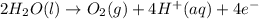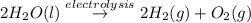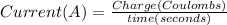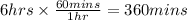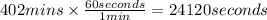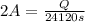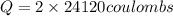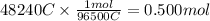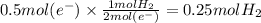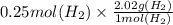Chemistry, 14.07.2019 10:00 nekathadon
In the electrolysis of water shown below, a current of 2 amps is applied to 180 ml of h2o(l) for 6 hours and 42 minutes. how many grams of h2(g) are formed? (faraday's constant = 96,500 c/mol)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1



Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
In the electrolysis of water shown below, a current of 2 amps is applied to 180 ml of h2o(l) for 6 h...
Questions


Business, 09.07.2019 12:30





Mathematics, 09.07.2019 12:30

Mathematics, 09.07.2019 12:30




Advanced Placement (AP), 09.07.2019 12:30