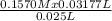Chemistry, 14.07.2019 09:00 Hjackson24
A25.00 ml sample of a solution of nitric acid, hno3, is titrated with a 0.1570 m solution of sodium hydroxide. the titration reaches the end point when 31.77 ml of the naoh solution has been added. what is the molarity of the nitric acid solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
A25.00 ml sample of a solution of nitric acid, hno3, is titrated with a 0.1570 m solution of sodium...
Questions

Mathematics, 27.10.2020 20:50

Mathematics, 27.10.2020 20:50

Mathematics, 27.10.2020 20:50



Mathematics, 27.10.2020 20:50




Mathematics, 27.10.2020 20:50

History, 27.10.2020 20:50

Chemistry, 27.10.2020 20:50

Mathematics, 27.10.2020 20:50

Mathematics, 27.10.2020 20:50

Mathematics, 27.10.2020 20:50



English, 27.10.2020 20:50

Medicine, 27.10.2020 20:50

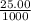 = 0.025 L
= 0.025 L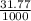 = 0.03177 L
= 0.03177 L