
Chemistry, 14.07.2019 09:00 baltazmapa629n
Isopropyl alcohol is mixed with water to produce a 37.0% (v/v) alcohol solution. how many milliliters of each component are present in 835 ml of this solution? (assume that volumes are additive.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Isopropyl alcohol is mixed with water to produce a 37.0% (v/v) alcohol solution. how many milliliter...
Questions


Mathematics, 06.05.2020 00:00

Mathematics, 06.05.2020 00:00

Mathematics, 06.05.2020 00:00


Mathematics, 06.05.2020 00:00



Computers and Technology, 06.05.2020 00:00

Mathematics, 06.05.2020 00:00


Biology, 06.05.2020 00:00


Mathematics, 06.05.2020 00:00


Mathematics, 06.05.2020 00:00

Mathematics, 06.05.2020 00:00

English, 06.05.2020 00:00

Mathematics, 06.05.2020 00:00

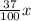 835 mL
835 mL


