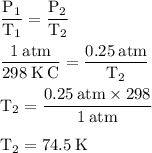
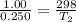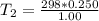Arigid cylinder contains 2.00 liters of gas at a temperature of 25°c. if the pressure of this gas is changed from 1.00 atmospheres to 0.250 atmospheres, what will be the new temperature (in kelvin, reported to three significant figures) of the gas? (the volume is constant.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1


Chemistry, 23.06.2019 08:00
Why is it important for scientists to review and repeat the work of other scientists? 1.a scientific theory must be tested three times before it is proven. 2.the scientific method only applies to repeated experiments. 3.an experiment may have had errors that the scientists didn't recognize. 4.the results of individual scientists may be influenced by bias. 5.an experiment must be performed twice before the data can be analyzed.
Answers: 3

Chemistry, 23.06.2019 10:20
Determine the mass of the object below with accuracy and to the correct degree of precision. a. 324.2 g b. 324 g c. 324.30 g d. 324.25 g
Answers: 3
You know the right answer?
Arigid cylinder contains 2.00 liters of gas at a temperature of 25°c. if the pressure of this gas is...
Questions


Mathematics, 09.10.2019 22:00



Mathematics, 09.10.2019 22:00





Biology, 09.10.2019 22:00



Social Studies, 09.10.2019 22:00


Business, 09.10.2019 22:00


Advanced Placement (AP), 09.10.2019 22:00

Social Studies, 09.10.2019 22:00

Mathematics, 09.10.2019 22:00

History, 09.10.2019 22:00

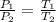
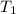 =25+273=298K
=25+273=298K
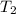 = ?
= ?
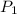 = 1.00 atm
= 1.00 atm
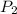 = 0.250 atm
= 0.250 atm