Chemistry, 14.07.2019 07:30 correafelix7
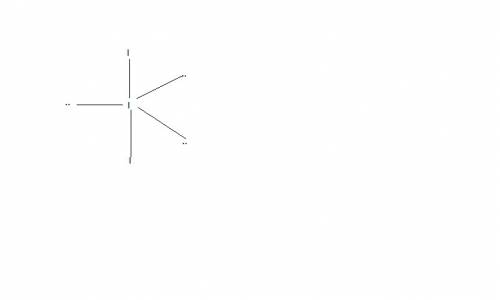
Which choice(s) include(s) d-orbital contribution in the hybridization scheme: pcl3, no3─, i3─, h2se ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
Which choice(s) include(s) d-orbital contribution in the hybridization scheme: pcl3, no3─, i3─, h2s...
Questions


French, 16.12.2020 03:40



English, 16.12.2020 03:40

Mathematics, 16.12.2020 03:40

Mathematics, 16.12.2020 03:40

Computers and Technology, 16.12.2020 03:40

English, 16.12.2020 03:40

Mathematics, 16.12.2020 03:40

Biology, 16.12.2020 03:40



Chemistry, 16.12.2020 03:40



Mathematics, 16.12.2020 03:40

Mathematics, 16.12.2020 03:40

Mathematics, 16.12.2020 03:40

Physics, 16.12.2020 03:40