
Chemistry, 14.07.2019 06:00 miathegreat
In a chemical reaction that takes place at a fixed pressure and volume, the enthalpy change (δh) is –585 kj/mol. will this reaction result in an increase or a decrease in temperature?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
In a chemical reaction that takes place at a fixed pressure and volume, the enthalpy change (δh) is...
Questions

Mathematics, 19.10.2020 04:01


Mathematics, 19.10.2020 04:01



Spanish, 19.10.2020 04:01

English, 19.10.2020 04:01




Mathematics, 19.10.2020 04:01

Mathematics, 19.10.2020 04:01

English, 19.10.2020 04:01



Mathematics, 19.10.2020 04:01


Physics, 19.10.2020 04:01


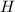 is negative it means an exothermic reaction where the heat is lose so the temperature decreases.
is negative it means an exothermic reaction where the heat is lose so the temperature decreases.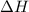 for the reaction comes out to be positive. The energy is absorbed and hence the temperature of the surroundings decrease.
for the reaction comes out to be positive. The energy is absorbed and hence the temperature of the surroundings decrease.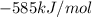 , the tempearture increase.
, the tempearture increase.

