

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
You know the right answer?
Chem ! using the average atomic mass, calculate the number of atoms present in 1.2 ml of liquid mer...
Questions



Biology, 07.12.2020 02:40


Mathematics, 07.12.2020 02:40


Mathematics, 07.12.2020 02:40


English, 07.12.2020 02:40

Health, 07.12.2020 02:40

Advanced Placement (AP), 07.12.2020 02:40



Mathematics, 07.12.2020 02:40


Biology, 07.12.2020 02:40



Mathematics, 07.12.2020 02:40

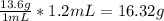
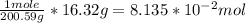
![\frac{6.022*10^{23}atoms}{1mole} *[8.135*10^{-2}mol]=4.90*10^{22}atoms](/tpl/images/0087/4847/f8efe.png)
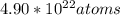 present.
present.

