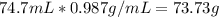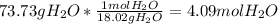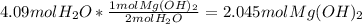Chemistry, 14.07.2019 04:30 haileyjones732
How many moles of magnesium hydroxide are needed to produce 74.7 milliliters of water, if the density of water is 0.987 g/ml? show all steps of your calculations as well as the final answer. hno3 + mg(oh)2 → h2o + mg(no3)2?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1


Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
How many moles of magnesium hydroxide are needed to produce 74.7 milliliters of water, if the densit...
Questions



English, 15.02.2022 04:40






Computers and Technology, 15.02.2022 04:40

Computers and Technology, 15.02.2022 04:40



English, 15.02.2022 04:40




Mathematics, 15.02.2022 04:40


Chemistry, 15.02.2022 04:40