
Chemistry, 14.07.2019 04:00 gilbert325
If 3.5 moles of nitrogen monoxide (no) react with 6.0 moles of oxygen gas (o2), how many moles of the product can be formed and how many moles of the excess reactant will be left over when the reaction is complete? show all of your work. unbalanced equation: no + o2 “yields”/ no2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
If 3.5 moles of nitrogen monoxide (no) react with 6.0 moles of oxygen gas (o2), how many moles of th...
Questions

Mathematics, 01.11.2020 08:40

Mathematics, 01.11.2020 08:40


Mathematics, 01.11.2020 08:40

Mathematics, 01.11.2020 08:40

Mathematics, 01.11.2020 08:40


Mathematics, 01.11.2020 08:40

Social Studies, 01.11.2020 08:40


Mathematics, 01.11.2020 08:40

English, 01.11.2020 08:40

Biology, 01.11.2020 08:40


English, 01.11.2020 08:40

Law, 01.11.2020 08:40

Mathematics, 01.11.2020 08:50

Mathematics, 01.11.2020 08:50


Mathematics, 01.11.2020 08:50

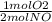 x 3.5 mol NO
x 3.5 mol NO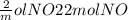 x 3.5 mol NO
x 3.5 mol NO

