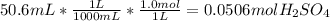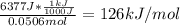Chemistry, 14.07.2019 00:00 BallerAlert1644
A101.2 ml sample of 1.00 m naoh is mixed with 50.6 ml of 1.00 m h2so4 in a large styrofoam coffee cup; the cup is fitted with a lid through which passes a calibrated thermometer. the temperature of each solution before mixing is 21.45 °c. after adding the naoh solution to the coffee cup and stirring the mixed solutions with the thermometer, the maximum temperature measured is 31.50 °c. assume that the density of the mixed solutions is 1.00 g/ml, that the specific heat of the mixed solutions is 4.18 j/(g·°c), and that no heat is lost to the surroundings

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
A101.2 ml sample of 1.00 m naoh is mixed with 50.6 ml of 1.00 m h2so4 in a large styrofoam coffee cu...
Questions


Biology, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00


English, 10.12.2020 14:00



Mathematics, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00


Mathematics, 10.12.2020 14:00

Health, 10.12.2020 14:00


Mathematics, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

English, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00

English, 10.12.2020 14:00


Mathematics, 10.12.2020 14:00


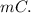 Δ
Δ
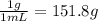
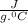
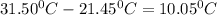
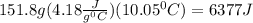
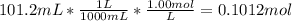 NaOH
NaOH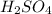 =
=