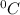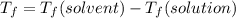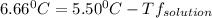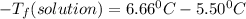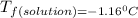Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
Calculate the freezing point of a solution made from 22.0 g of octane (c8h18) dissolved in 148.0 g o...
Questions


Chemistry, 19.12.2019 21:31




Mathematics, 19.12.2019 21:31







Mathematics, 19.12.2019 21:31

Computers and Technology, 19.12.2019 21:31






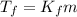
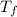 is the change in the freezing point of the solvent.
is the change in the freezing point of the solvent.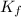 of benzene is 5.12
of benzene is 5.12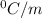
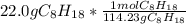 = 0.193 mol
= 0.193 mol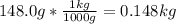
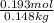
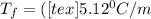 ) (0.130 m) " alt=" 5.12^{0}C/m" />) (0.130 m) " />
) (0.130 m) " alt=" 5.12^{0}C/m" />) (0.130 m) " />