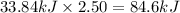Nitrogen dioxide, no2( g. hf = 33.84 kj/mol), is decomposed according to the following reaction: what is the enthalpy change when 2.50 mol of nitrogen dioxide decomposes? 13.5 kj of energy released 13.5 kj of energy absorbed 84.6 kj of energy released 84.6 kj of energy absorbed

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Nitrogen dioxide, no2( g. hf = 33.84 kj/mol), is decomposed according to the following reaction: wh...
Questions

Mathematics, 05.02.2020 07:51

Mathematics, 05.02.2020 07:51

History, 05.02.2020 07:51



Physics, 05.02.2020 07:51

Mathematics, 05.02.2020 07:51



Biology, 05.02.2020 07:51




Social Studies, 05.02.2020 07:51


Social Studies, 05.02.2020 07:51


Geography, 05.02.2020 07:51


Chemistry, 05.02.2020 07:51

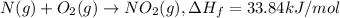
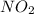 is formed = 33.84 kJ
is formed = 33.84 kJ