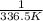Chemistry, 13.07.2019 21:30 thomasg185
The vapor pressure of ethanol is 400. mmhg at 63.5°c. its molar heat of vaporization is 39.3 kj/mol. what is the vapor pressure of ethanol, in mmhg, at 34.9°c? (r = 8.314 j/k • mol)

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3
You know the right answer?
The vapor pressure of ethanol is 400. mmhg at 63.5°c. its molar heat of vaporization is 39.3 kj/mol....
Questions


Mathematics, 20.09.2020 09:01

History, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01

World Languages, 20.09.2020 09:01

Computers and Technology, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01



Health, 20.09.2020 09:01


Biology, 20.09.2020 09:01






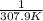 -
-